Home
| About
| Mine Tracker
| RSS
| Footer
▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄▄ █▄▄░▄▄█░▄▄▀█░▄▄▀█▀▄▀█░▄▄███░▄▄▀█░▄▄▀█░▄▀███▄▄░▄▄█░▄▄▀█░▄▄▀█▀▄▀█░▄▄█░▄▄▀█░▄▄▀██▄██░███▄██▄░▄█░██░ ███░███░▀▀▄█░▀▀░█░█▀█░▄▄███░▀▀░█░██░█░█░█████░███░▀▀▄█░▀▀░█░█▀█░▄▄█░▀▀░█░▄▄▀██░▄█░███░▄██░██░▀▀░ ███░███▄█▄▄█▄██▄██▄██▄▄▄███▄██▄█▄██▄█▄▄██████░███▄█▄▄█▄██▄██▄██▄▄▄█▄██▄█▄▄▄▄█▄▄▄█▄▄█▄▄▄██▄██▀▀▀▄ ▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀▀
FSMA Final Rule on Requirements for Additional Traceability Records for Certain Foods
https://www.fda.gov/food/food-safety-modernization-act-fsma/fsma-final-rule-requirements-additional-traceability-records-certain-foods
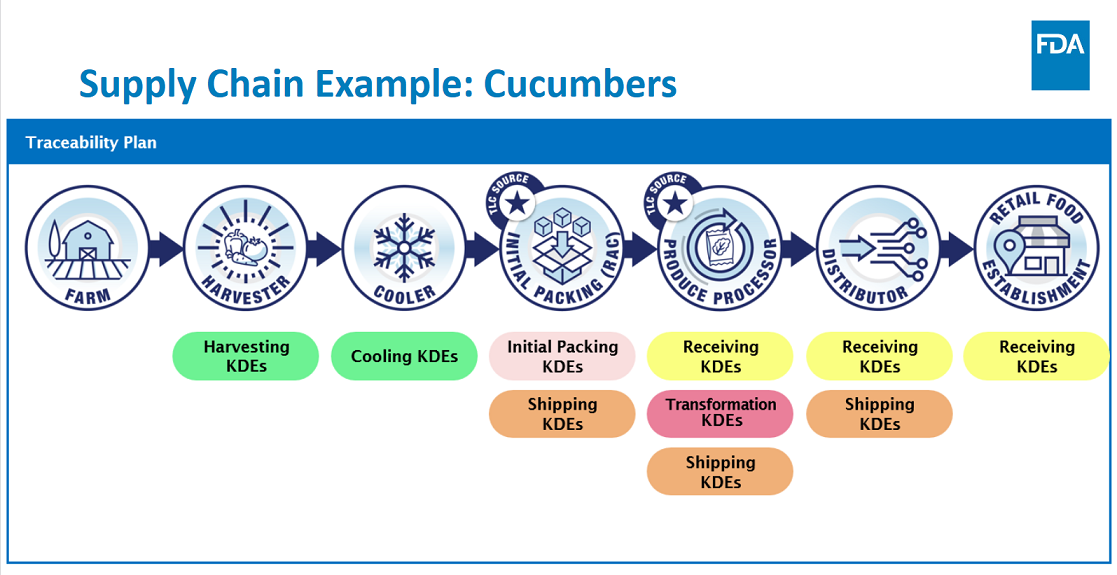
At the core of this rule is a requirement that persons subject to the rule who manufacture, process, pack, or hold foods on the FTL, maintain records containing Key Data Elements (KDEs) associated with specific Critical Tracking Events (CTEs); and provide information to the FDA within 24 hours or within some reasonable time to which the FDA has agreed.
https://www.fda.gov/food/food-safety-modernization-act-fsma/fsma-final-rule-requirements-additional-traceability-records-certain-foods

At the core of this rule is a requirement that persons subject to the rule who manufacture, process, pack, or hold foods on the FTL, maintain records containing Key Data Elements (KDEs) associated with specific Critical Tracking Events (CTEs); and provide information to the FDA within 24 hours or within some reasonable time to which the FDA has agreed.
Integrity and Assurance of Food Supply Networks
https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/350726/elliot-review-final-report-july2014.pdf
A National Food Crime Prevention Framework
https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/350726/elliot-review-final-report-july2014.pdf
A National Food Crime Prevention Framework
History of Food Traceability
https://www.researchgate.net/publication/325247189_History_of_food_traceability
In the past the food industry has had its fair share of scandals, accidents, and incidents. It must be pointed out that reported food scares were not always associated with microorganisms; many of them were connected to new technology, environmental pollution or changes in co-product management. For example the food colorant (tartrazine and amaranth) incident reported in mid-1980 in UK; mercury poisoning in oranges reported in 1979; mercury poisoning in fish reported in 1970; radioactivity in lamb reported in 1986; glass, pin and caustic soda found in baby food product reported in UK in 1989 which resulted in the recall of 100 million jars off the shelves and repackaging of another 60 million. These incidents are very much in the memories of the general public.
https://www.researchgate.net/publication/325247189_History_of_food_traceability
In the past the food industry has had its fair share of scandals, accidents, and incidents. It must be pointed out that reported food scares were not always associated with microorganisms; many of them were connected to new technology, environmental pollution or changes in co-product management. For example the food colorant (tartrazine and amaranth) incident reported in mid-1980 in UK; mercury poisoning in oranges reported in 1979; mercury poisoning in fish reported in 1970; radioactivity in lamb reported in 1986; glass, pin and caustic soda found in baby food product reported in UK in 1989 which resulted in the recall of 100 million jars off the shelves and repackaging of another 60 million. These incidents are very much in the memories of the general public.
The impact of improved traceability on the safety of food
https://www.lrfoundation.org.uk/en/news/impact-traceability-food-safety/
In a new report RS Standards have reviewed the impact of improved traceability on the safety of food, looking at current initiatives that could provide a basis for a roadmap for future developments.
https://www.lrfoundation.org.uk/en/news/impact-traceability-food-safety/
In a new report RS Standards have reviewed the impact of improved traceability on the safety of food, looking at current initiatives that could provide a basis for a roadmap for future developments.